The only foreign language that I claim to know is Japanese.
I started studying Japanese in elementary school and took courses throughout middle and high school as well. Simple Japanese books, everyday conversations and Japanese
YouTubers are fine for the most part, but vocabulary is still a problem for me. While at work the other day, I decided to practice some new terms I read on a blog post.
Papers covered in words like this are scattered all over my room. Sometimes the need to write just
gets to you, you know? Staring at the scribbled words, I suddenly thought, what
is this stuff I’m writing with anyways?
Ignoring the fancy colors, black pen
ink is a relatively simple composition made of carbon black suspended in drying
oils, alcohols or petroleum products [1]. Carbon black is carbon powder
obtained by burning organic matter. This type of black ink has been around for
thousands of years, and I use another form of the same stuff while doing Japanese
calligraphy. East Asian calligraphy ink is traditionally made of pine soot (carbon black) mixed with bindings and herbs. This page provides a more in-depth description
of how sumi, or a Japanese ink stick, is made.
Fig. 2: Carbon black sample molecular structure (Bruno Glaser)
Fig. 2: Carbon black sample molecular structure (Bruno Glaser)
So why is carbon black... black? Writer Maggie Koerth-Baker
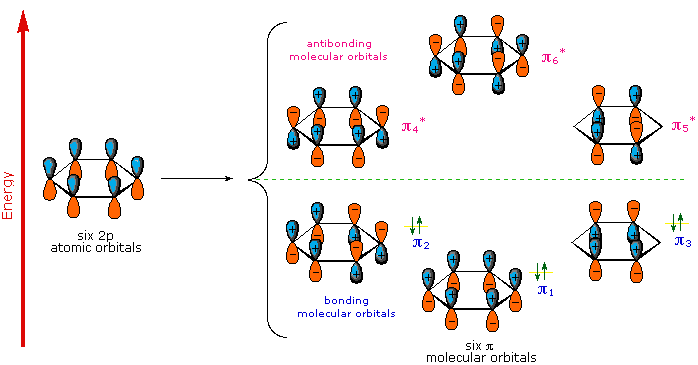
does a good job of describing this. In organic chemistry, pi-conjugated
systems, or systems where electrons of sp2 hybridized atoms are delocalized in combined p-orbitals, can exhibit a wide range of light absorption
and emission properties. This is because of the many possible bonding and
antibonding p-orbital orientations within the conjugated system.
When we talked about why excited metals emit colored light back in The Dazzling Chemistry of Fireworks, we mentioned that the Fermi
level of metals is located within an electronic band, meaning that electron
transitions between the highest occupied energy level and other higher energy
electron states are possible over a range of values. The many available higher
energy electron states are not unlike the many possible organizations of bonding
and antibonding orbitals in pi-conjugated systems. An incident photon on a pi-conjugated
system can upset the shared p-orbital system into occupying one of the possible
antibonding arrangements, later to reemit the light energy at lower, sometimes non-visible spectrum, wavelengths.
Pi-conjugated systems that include more atoms result in more complex molecular
orbital states. And when conjugated units of varied length exist together in a material, then on the whole even more electronic states are possible! All of these electron transitions cooperate to make the familiar black ink of pens (graphite pencils too, just think
about it).
If you enjoyed this post and those I've written before, share your thoughts in the comments
to let me know!


No comments:
Post a Comment