Yesterday was the Fourth of July, and millions of people all over America gathered in parks and along waterways to witness one of the most dazzling examples of ancient science; fireworks. The invention of gunpowder in China ca. 600-900 CE resulted in the creation of the first fireworks, later buffed up to the colorful sky-flowers we know today by Italian fireworks makers in the 1830s [1]. While waiting for a fireworks display at the Philadelphia Museum of Art, I found myself wondering how is it that we humans have come to create this technology. The first step was to devise a method of controlling highly exothermic and expansive (due to gas formation) chemical reactions and initiate them on purpose through the use of chemical oxidizers (originally saltpeter, KNO3) that contribute oxygen to combustion reactions, allowing them to burn in containers without oxygen flow or even in vacuums. But the role of gunpowder in history has had many uses beyond fireworks and isn’t what makes this specific variety of chemical explosions so addicting. What gives fireworks their characteristic hues are the metal ions that exist along with the crafted gunpowder in fireworks shells. Italian fireworks makers discovered that the use of KClO3 rather than saltpeter could increase the temperature of the combustion reaction, allowing for the light-emitting potential of metal ions to be unleashed [2]. When calculating the standard enthalpy of reaction for the following decompositions;
1. 2KNO3 --> K2O + N2 + 2.5O2
2. 2KClO3 --> 2KCl + 3O2
it can be observed that the decomposition of KClO3 is more
exothermic than that of KNO3 (625 kJ/mol for KNO3 and -89.6 kJ/mol for KClO3
based on standard enthalpies of formation found on Wikipedia), the former also
generating 0.5 more moles of O2 gas than the latter. The extra oxygen gas
contributes to the exothermic nature of the combustion through the formation of CO2 and SO2 gas (-393.5 kJ/mol for CO2 and -296.81 kJ/mol for SO2 based on Wikipedia
values), the same source of energy from which coal power plants operate,
according to the following equations:
The color that a given metal salt produces when heated is
characteristic of the metal element and can be calculated based on the
following equations [3,4];
5. E=hv
6. c=lv (v=fl modified for light)
7. E=hc/l (from equations 5. and 6.)
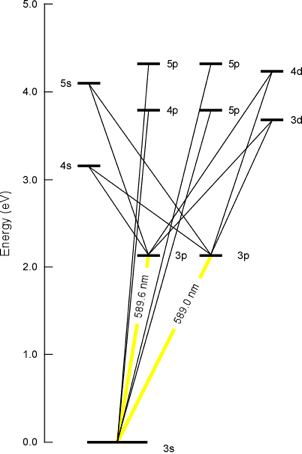
From the above electron orbital energy diagram for sodium, it
can be seen that the transition between the highest occupied molecular orbital (HOMO), the 3s orbital, and the lowest unoccupied molecular orbitals (LUMO),
the 3p set, corresponds to an emission of light in the 589nm wavelength region,
or yellow light. This fact is experimentally verified since the yellow color of
fireworks is often produced by sodium salts. It is in this fashion that the colors
each metal element produces can be understood. So why metals? Well, the
emission of light in various visible colors is reliant on electron energy state transitions as we just discussed, and electron band structure properties for metals
tell us that the Fermi level, or highest occupied energy level (HOMO energy level), lies in the
middle of an electron band in metals, meaning that there are higher electron
energy levels to which metal electrons may be excited [5]. So metals are used
simply because in our universe these are the elements that are capable of
light-producing electron transitions.
Of course other factors must go into fireworks to achieve
such controlled chemical acrobatics as producing tailed explosions or glittery
rain, but with bang and color you have the basic makings of this recreational
technology that is also a marvel of modern chemistry.
I plan to do experiments inspired by daily events or questions that arise, so comment if this post has brought up any topics you would like to hear more about or want to see experiments on. Thanks!
I plan to do experiments inspired by daily events or questions that arise, so comment if this post has brought up any topics you would like to hear more about or want to see experiments on. Thanks!

I'm glad I found this web site, I couldn't find any knowledge on this matter prior to.Also operate a site and if you are ever interested in doing some visitor writing for me if possible feel free to let me know, im always look for people to check out my web site.
ReplyDeleteclick here