Ever wonder why it is that we use soap to clean our cars,
dishes, bodies and hands? Aside from dyes, perfumes and other additives, soap contains
molecules called surfactants. Surfactants are complex molecules that have
hydrophobic "tail" regions consisting of long carbon chains and hydrophilic "head" regions that are charged or polar. Some of the oldest soaps were made by
heating water washed through wood ash (called caustic soda, lye, or sodium
hydroxide) with a fat source [1]. Fat sources can be triglycerides from animal fat,
fatty acids from plants or other sources of similar molecules often possessing a
carboxylic acid (hence the term "fatty acid") or fatty acid-derived ester
group as in triglycerides. The chemical reaction that results is called "saponification" and involves the cleavage of triglyceride fatty acids by hydroxide ions forming
glycerol and neutralization of the fatty acids to form salts of the fatty acids.
Fig. 1: Saponification mechanism for fatty acid-derived esters in triglycerides (University of Malta)
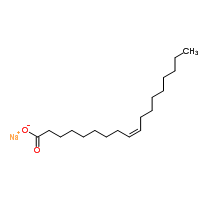
An example
of the type of fatty acid salt that results from saponification is sodium
oleate;
Fig. 1: Saponification mechanism for fatty acid-derived esters in triglycerides (University of Malta)
Fig. 2: Sodium oleate (Exporters India)
This surfactant molecule is formed by reacting sodium
hydroxide with oleic acid, better known as olive oil. So how do these
surfactants help to clean dishes? Let’s think about what types of foods are the
worst to clean after cooking dinner. Sugary syrups from cranberry sauce, jams and
the like are a breeze to clean; just rinse the dishes and bam, they’re clean. This
is because sugars are water soluble. The oily pan used to fry meat, however, is
a mess and gives your hands that slick feeling everybody hates. Oil is
hydrophobic and will likely stick to your pan better than it does to the water
running over it. Here is where soap helps. Above a critical concentration, surfactants
tend to form clumps in water called "micelles" so that all of the hydrophobic
tails are collected inward (a bilayered micelle is a lysosome, and a lysosome
with its hydrophobic tails pointed outward containing a water layer of varying
thickness is a bubble).
Fig. 3: Surfactant organization forms in water (Wikipedia)
Exactly at what concentration these micelles form is dictated by thermodynamics in balancing the loss of entropy from organizing the surfactant molecules against the higher energy of associating water and the hydrophobic tail regions [3]. When soap is applied to the oily pan surface, the hydrophobic tails embed in the oil and form it into small droplets. The oil is then escorted by the hydrophilic head regions into the surrounding water and down the drain when rinsed. It is this ability to make hydrophobic substances pseudo-hydrophilic that makes soap so useful to us. What about washing cars and our bodies. While the circumstances are radically different and accordingly warrant different surfactants, the mechanism is the same. Soap helps to escort away hydrophilic bits in dirt, grime and bird poop on cars and dead cells and human oils on our bodies, helping to keep things in line with our modern sense of cleanliness.
Fig. 3: Surfactant organization forms in water (Wikipedia)
Exactly at what concentration these micelles form is dictated by thermodynamics in balancing the loss of entropy from organizing the surfactant molecules against the higher energy of associating water and the hydrophobic tail regions [3]. When soap is applied to the oily pan surface, the hydrophobic tails embed in the oil and form it into small droplets. The oil is then escorted by the hydrophilic head regions into the surrounding water and down the drain when rinsed. It is this ability to make hydrophobic substances pseudo-hydrophilic that makes soap so useful to us. What about washing cars and our bodies. While the circumstances are radically different and accordingly warrant different surfactants, the mechanism is the same. Soap helps to escort away hydrophilic bits in dirt, grime and bird poop on cars and dead cells and human oils on our bodies, helping to keep things in line with our modern sense of cleanliness.
So there you have it, a summary of how we utilize soap. If you
guys have any topics you would like to hear about or this article tickled your
fancy, leave a comment so I can hear your thoughts.


Oooh ooh ooh talk about colors and how we perceive colors differently. I'm pretty sure you're going to have a hard time trying to put a chemical formula into that one :). ~ Lilia
ReplyDelete