With a horrible heat wave hitting the Philadelphia area,
it’s good to think cool thoughts. Already feeling the heat last night, I left a
coconut water in the freezer with the intent to drink it but forgot and so took
it to work this morning frozen solid. I figured since it’s so hot outside and
the metal can is a good conductor, it’d probably melt pretty quickly. And while
the ice in immediate contact did melt, the inside remained frozen and I had to
cut the top open with scissors to eat it. Before I figured this out, the can
had already shed a puddle at my desk. Have you ever wondered why it is that
cold things "sweat."
Fig. 1: My favorite coconut juice brand, Foco (pinstopin.com)
Fig. 1: My favorite coconut juice brand, Foco (pinstopin.com)
Most of us are familiar with the concept of condensation,
having learned about the water cycle in elementary school. We are commonly
taught in elementary that water exists as vapor at hot temperatures, condenses
to liquid as the temperature drops and eventually expands (not condenses, as
ice has a lower density than water due to hydrogen bonding) into ice as the
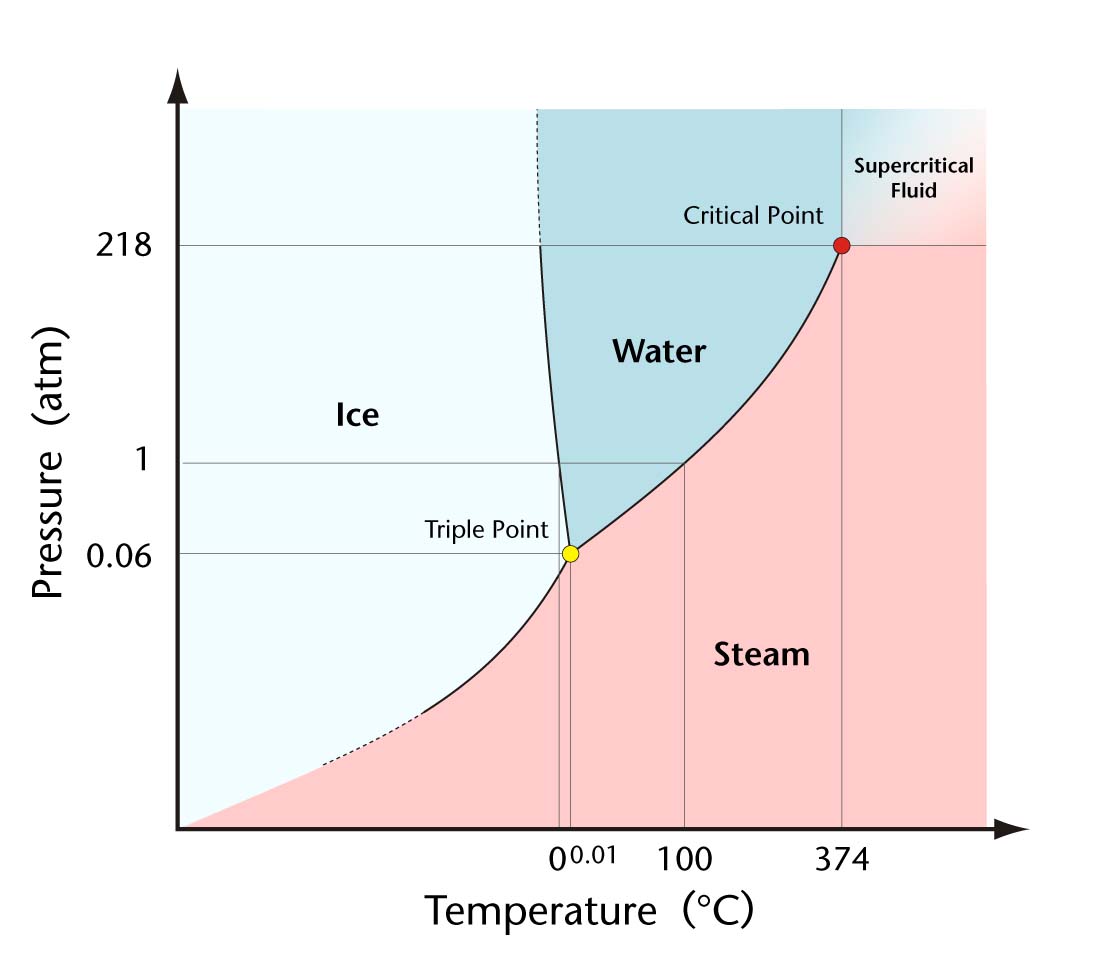
temperature drops further. In high school, we learn about the ideal gas law and
how pressure also affects phase transitions, yielding the phase diagram.
Fig. 2: Phase diagram for water (myhomeimprovement.org)
So from this standpoint, we are all familiar with why cold
things "sweat." What else is there to it? While the basic principles stand,
there are some other viewpoints from which we can view this phenomenon.
Phase transitions can be viewed as being an equilibrium
process, as is demonstrated by the fact that an ice and water mix maintains a
0°C temperature. In such a mix, the ice melting and the water freezing are
competing processes that are controlled by environmental factors; if you cool
the mix the ice expands, but if heated the ice melts. Additionally, the entire
mix must either become ice or water only before the temperature can deviate
significantly from the equilibrium temperature of 0°C. What’s cool about this
process is that if you track the energy entering the ice and water mix, say a
glass of iced coconut water (let's treat this as an ideal glass of pure iced water), we can predict the corresponding
phase transitions based on molecular kinetics.
When bonds are formed, whether strong or weak, we know that
energy is released as heat. The reverse is true as well, breaking bonds
requiring energy. The direction of bond energy transfer can be simplistically remembered
taking into account the conservation of energy in a two molecule one-dimensional
collision. Say two water molecules are moving towards each other and stick together upon impact. Where did the kinetic energy go? Ignoring molecular vibrations, the energy
had to have been released as work, or heat. In order to separate the water
molecules, we need to get them to move apart, a.k.a. add work, or heat, to yield
kinetic energy. In our glass of iced water, this sort of energy transfer is
happening extremely fast and on a large scale, one that can be described by Le
Chatelier’s principle since the ice and water form an equilibrium.
Now let’s put the iced water outside on a hot summer Philadelphia
day. From experience, we know that the ice will melt and the water will become unappealingly warm. If we track the direction of energy transfer, the higher
energy hot air must be donating energy to the lower energy iced water simply because
this is the default direction of energy transfer in our universe according to
the Second Law of Thermodynamics. The added energy must translate into kinetic
energy as temperature is positively correlated to molecular kinetic energy. From
our two water molecule system we know that a decrease in water molecule association
is predicted, favoring water over ice and vapor over water. This manifests as the
ice melting and the water warming and eventually evaporating.
What has been so far described, however, is only focused on
the iced water itself. Let’s change our basis to focus on the hot air along the
iced water glass instead (assume the water glass does not hamper kinetic energy
exchange between air and iced water). Hot air carries a lot of water since at
higher temperatures water enters the vapor phase preferentially according to Le
Chatelier’s principle. From the viewpoint of the air, the cold iced water is
pulling kinetic energy from it, accordingly cooling the air within a certain
range of the glass. Plugging this information back into our two molecule
system, the energy must be afforded by reducing the molecular kinetic energy of
the air, increasing the probability of water existing in associated groups, i.e.
water. And this is why a cold drink sweats in the summer.
Since school is starting up again, I will not be able to
post as frequently as I have been during the summer. I will try to post at
least once a week, and will probably be doing so during the weekend since this
is when time is most available. Please have patience with me on this, and as
always thanks for reading!


I found this is an informative and interesting post so i think so it is very useful and knowledgeable. I would like to thank you for the efforts you have made in writing this article.
ReplyDeletehttps://www.idealglass.studio/
Great knowledge, do anyone mind merely reference back to it
ReplyDeleteidealglass.studio